Cardiac Imaging
Summary:
Multiphoton microscopy has been demonstrated as a versatile tool in the life sciences for many structural and functional applications both in-situ and in-vivo, including electrophysiology, calcium signalling, blood flow and immune cell trafficking in the heart in vivo. Despite the analytic power of such devices, and their increased availability both as commercially and as a custom-built device, their uptake in biology labs remains relatively low in comparison to the sister technology of confocal microscopy. This is in part due to the size, cost, and perceived complexity of purchasing and maintaining the most crucial piece of equipment for two-photon microscopy; the laser. While expensive, bulky Ti:Sapphire lasers remain the most commonly used laser for two-photon applications. There is an increasing need for low-cost, compact commercially available turnkey solutions capable of delivering higher average power at wavelengths >1000 nm, where the traditional tuneable Ti:Sapphire system struggle to deliver. This is demonstrated by electrophysiological measurements in excitable tissues, particularly cardiac and neural tissue. This frequently involves recording membrane potentials and calcium transients via multiphoton processes. For this purpose, a broad range of voltage and calciumsensitive fluorophores have been specifically developed. However, these processes are in the second IR imaging window (1 – 1.3 μm), wavelengths where the power of Ti:Sapphire systems fall off dramatically (Figure 1). Here we demonstrate the use of a low cost commercially available turnkey laser system, from Chromacity. It is capable of delivering high peak and average powers at the sample plane of a two-photon microscope.
 Figure 1. Emission Spectra of Ti:Sapphire and Chromacity 1040 lasers.
Figure 1. Emission Spectra of Ti:Sapphire and Chromacity 1040 lasers.
The laser was able to perform equally well relative to a commercially available solid-state Ti:Sapphire laser at 1040 nm. It was easily inserted into an existing two photon microscope. The high power demands of deep tissue optical electrophysiological imaging were met using this laser; The Chromacity 1040 could record action potentials at deeper layers than Ti:Sapphire.
Introduction:
Two-photon imaging for in vivo applications has been shown to achieve high-resolution imaging and execute functional measurements within neuroscience. Such functional measurements are also being performed in other excitable media such as cardiac muscle, which exhibits even greater absorption and scattering. Despite the relatively high tissue penetration depths afforded by this technique there remain fundamental limits to the depth of imaging possible, which are a function of the detection characteristics of the microscope, the optical properties of the tissue being imaged, and more importantly the pulsed laser source. The increased requirement for deep-tissue in vivo imaging has also spurred the development of genetically encoded sensors for red-shifted imaging. The signal-to-noise requirements for fluorescent electrophysiological recordings are much lower than that required to resolve a detailed structural image. Therefore, during purely electrophysiological measurements, the ability to deliver sufficient power to a confined region deep within certain tissue types becomes more important than resolving individual structural features. This is particularly true in electrically well-coupled tissue such as cardiac muscle, hence, the need for suitable optical powers being delivered to the sample plane.
Optical Setup:
A schematic of the optical layout is shown in Figure 2. An ultrafast ytterbium fibre laser from Chromacity capable of delivering 100 fs pulses at 80 MHz repetition rate, was coupled to a Zeiss LSM510 laser scanning microscope system. Laser output power was manually controlled using a Chromacity power attenuation module. Beam divergence was compensated for using a divergence – adjustable beam expander. The beam was focused into the LSM510 scan head. The use of a 90° flip-mount and mirror allowed the beam path to be switched rapidly between the Chromacity laser and a tuneable Ti:Sapphire laser.
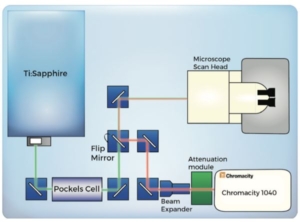
Figure 2. Laser and microscope optical setup. Beam path and
intermediate optics for Ti:Sapphire and Chromacity 1040 laser is
shown above.
Isolated perfused heart preparation:
Hearts from three animals were used in the study. Hearts were bolus loaded with 25 μl of di-4-ANEPPS (2 mM stock) over a 10 min period. Imaging of cardiac structures was performed using a 40x mag, 1.0 NA objective lens. Two bi-alkali photomultiplier tubes (PMT) were used to capture the dual emission from di-4-ANEPPS. PMT 1 collected emitted light between 510-560 nm while PMT 2 collected the longer 590-650 nm emission band. A series of XY images were recorded using each laser to form an image stack (z-stack). Afterwards, rapid line scanning at 1 kHz was used to provide sufficient bandwidth to record optical action potentials. A ratio of both PMT signals was then taken to reduce motion artifact and baseline drift. This process was repeated sequentially, in 50 μm steps, at increasing transmural tissue depths from 50 to 250 μm below the epicardial surface.
Results:
Pulse characteristics were determined initially using autocorrelation techniques. The autocorrelation for the Ti:Sapphire, yielded a pulse duration of 120 fs. The design of the Chromacity 1040 nm system features a movable diffraction grating, resulting in pre-chirping of the pulse to compensate for dispersion effects through the microscope optics and maximise the fluorescence signal at the sample plane. Once the beam was aligned with the microscope scan head the diffraction grating was adjusted to yield the maximal fluorescence from a test sample of 500 μm fluorescent beads.
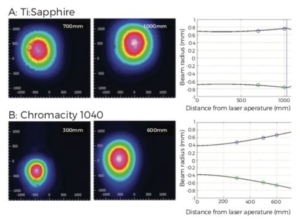
Figure 3. Laser beam profiles for Ti:Sapphire (A) and Chromacity 1040
(B). Blue lines on plots indicate relative position of focusing lenses for
each laser beam path. Initial measurements showed the beam waist of
the Chromacity 1040 to be narrower than the Ti:Sapphire but diverging
much quicker. This divergence was compensated for in the final set up
using a divergence adjustable beam expander.
Laser beam profiling measurements demonstrated that the beam waist of the Ti:Sapphire (Figure 3A) was roughly double that of the Chromacity 1040 (Figure 3B). As a consequence, the Chromacity 1040 beam diverged more rapidly than the Ti:Sapphire along its beam path. The Chromacity 1040 optical path therefore included a divergence adjustable beam expander. This yielded two photon- excited fluorescent signals with intensity matching the Ti:Sapphire at 1040 nm (PMT gain and average power at sample plane matched). This was confirmed from z-stack images taken through the left ventricular epicardial surface at 15 mW average power (Figure 4 and 5).
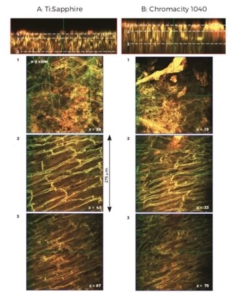
Figure 4. Z-stack structural images from adjacent regions of the
epicardial surface of mouse left ventricle using Ti:Sapphire (A) and
Chromacity 1040 (B). Average laser power at sample and PMT gain were
matched in each. Top panels show y-z orthogonal view through the
transmural wall, with x-y images from 3 separate planes (indicated by
white dashed lines).
Due to the length of each scan, z-stacks for each laser were taken at adjacent regions of tissue to avoid any disparity in signal due to dye bleaching. The average signal from a defined imaging region was almost identical at each z-stack image plane for both lasers when power and wavelengthmatched (Figure 5B).
Optical action potentials recorded from a series of transmural depths with both lasers using rapid line scanning are shown in Figure 6A. Average power at the sample plane was relatively low (25 mW) close to the epicardial surface (50 μm z-depth).
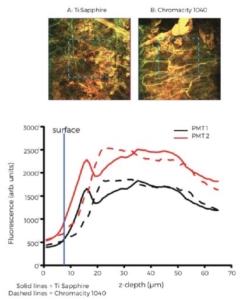
Figure 5. Mean fluorescence intensity with depth. Epicardial x-y
surface images from adjacent regions using the Ti:Sapphire (left)
and Chromacity 1040 (right). B Mean fluorescence intensity in zstack
images for each PMT channel (red, PMT 1, black PMT 2)
measured from the regions enclosed by the dashed blue box in A.
Blue vertical line indicates which image plane denotes the absolute
epicardial surface from each image z-stack. Both lasers at 1040n and
PMT gain matched.
Here both lasers performed equally well at 1040 nm, producing action potentials of comparable amplitude. From depths of 100- A 250 μm, power was increased on the Ti:Sapphire to maximum, yielding 60 mW at the sample plane. Figure 6B shows the drop in action potential amplitude with depth, to the extent that signal to noise was insufficient at 200 μm below the surface to record a usable action potential. Increasing the power of the Chromacity 1040 to 75 mW, 15 mW above the Ti:Sapphire power threshold at 1040 nm, allowed action potentials of sufficient signal to noise to be recorded as deep as 250 μm, without damaging the tissue sample.
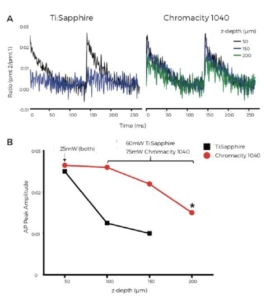
Figure 6. Higher average powers allow recording of deeper transmural
optical action potentials. An Optical action potential from 3 transmural
layers (50, 150 and 200μm below epicardial surface) using the
Ti:Sapphire (left) and Chromacity 1040 (right) at 1040nm (PMT gain
matched). A ratio of the signals from PMT channels 1 & 2 was used to
limit motion and baseline drift from photobleaching. B Max AP amplitude
at each layer using the Ti:Sapphire (black squares) and Chromacity 1040
(red circles). Ti:Sapphire power was maximal between 100-200μm and
was unable to resolve an action potential at 200μm (indicated by *).
Higher powers from the Chromacity 1040 overcame this problem.
Discussion:
Many system builders still opt for bundling microscopes with a tuneable, solid state Ti:Sapphire laser which, while offering flexibility, is bulky, costly to purchase and requires regular maintenance. Advances in fibre lasers in recent years has 0 seen a significant increase in the use of this technology as an efficient, low cost alternative to traditional solid-state lasers, albeit limited to single wavelengths. Low cost custombuilt fibre lasers for two-photon applications have been demonstrated for as little as $13,000, but remain within the realm of specialist users with the appropriate knowledge and skillset to construct them.
In our setup the Chromacity 1040 nm system can deliver >400 mW at the sample plane, whereas the Ti:Sapphire can only deliver 60 mW. While 400 mW is in excess of what biological tissue can tolerate, it is likely that increasing the power beyond 75 mW at deeper transmural tissue planes, shown here, would increase the range of imaging of the system even further than that reported here while remaining within the tolerable energy limits for intact cardiac tissue.
The flexibility of this laser system is illustrated by the relative ease with which it was coupled into the existing microscope scan head, without any prior knowledge of the optics within. The footprint of the system is low, occupying almost 2.5x less optical-table space than the Ti:Sapphire and requiring only a single panel-mounted control unit. In addition, the system is passively aircooled, eliminating maintenance costs and further reducing the overall footprint of the two-photon microscope.
Conclusion:
Significant improvements in transmural, deep tissue imaging of cardiac tissue can be gained through the use of a commercially available high-power ytterbium fibre laser at 1040 nm relative to existing Ti:Sapphire lasers. This laser platform offers ease of use and installation into a laser scanning microscope system and is suitable for new and existing microscopes alike.
Benefits:
Comparing the Chromacity 1040 laser to the commercially available solid-state Ti:Sapphire laser at 1040 nm the Chromacity 1040 was able to perform equally well and in some cases better. The Spark 1040 was easily able to meet the high power demands of deep tissue optical electro-physiological imaging. In this short demonstration it enabled recording of action potentials from 250 μm, whereas the Ti:Sapphire was only able to reach 200 μm. The Chromacity 1040 was able to deliver >400 mW at the sample plane, way in excess of the 75 mW used to obtain the recording of the action potentials at 250 μm. While 400 mW is in excess of what biological tissue can tolerate, we believe that increasing the power beyond 75 mW at deeper transmural tissue planes would increase the range of imaging of the system even further than the 250 μm while remaining within the tolerable energy limits for intact cardiac tissue.
The Chromacity 1040 can be coupled into an existing microscope scan head with relative ease, without any prior knowledge of the optics within. The footprint is almost 2.5x less air table space than the Ti:Sapphire and requires only a single panel-mounted control unit. In addition, the system is passively air-cooled, eliminating maintenance costs and further reducing the overall footprint of the two-photon microscope.
For users wishing to purchase an entirely new system the fiber laser option reduces space considerably and brings the total cost for a complete basic laser scanning two-photon system down to between £100,000 – 120,000, close to the price of a single Ti:Sapphire laser alone. Those with an existing microscope who require more power for deep tissue imaging at wavelengths >1000 nm could also consider this as an option thanks to the compact size of the Chromacity 1040 system.
Per maggiori informazioni sui prodotti correlati vai alla nostra pagina:



